- Waiting Chairs[10]
- Living Room Chairs[10]
- Office Chairs[1]
- Dining Chairs[7]
- Other Office Furniture[6]
- Coffee Tables[4]
- Furniture Legs[7]
- Hospital Chairs[7]
- Furniture Frames[7]
- School Sets[2]
- Contact Person : Ms. HUANG YING
- Company Name : Kaize Furniture Co., Ltd.
- Tel : 86-0750-6700113
- Fax : 0750-6700380
- Address : Guangdong,JIANGMEN,No. 51, Yong'an 2nd Industrial Zone, Huicheng, Xinhui District, Jiangmen, Guangdong, China (Mainland)
- Country/Region : China
- Zip : 529100
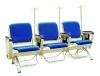
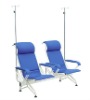
Good quality 3 seat blue comfor simple hospital drip chair/infusion chair
Product Name: Good quality 3 seat blue comfor simple hospital drip chair/infusion chair
Size: 2625L*650W*1800Hmm
Application areas: Medical care places.
Material Indication:
Chair-seat: High-quality cold-rolled sheet is pressed to form the shape and the surfaces are sprayed with epoxy powder after cid cleaning,rust-removal and phosphate coating.
Decorative Edge: Aluminum alloy is bending to form the shape and the surfaces are polished.
Arm/foot: High quality SS tube is welded with mould to form the shape and the surfaces are polished and then plated.
Beam: High-quality cold-rolled sheet is pressed and welded to form the shape and the surfaces are sprayed with epoxy powder after acid cleaning, rust-removal and phosphate coating.
Notes:
1. Can selectable from single seat to 5 seat.
2.Leather cushion: Selectable for separable or connected seat cushion.
Seat's cotton fabric:High density formed foam.
Seat's leather :High quality PU pro-environment leahter.
Advantage:
1. Firmly & Durable2. Pro-environment & Easy assembly 3. Modern & Simple Style
4. Personality design & Attentive service
5. Good reputation & timely delivery
6. Excellent quality and very competitive price
More information and questions pls contact me.
Good quality 3 seat blue comfor simple hospital drip chair/infusion chair